Preparation of a home-made Ag/AgCl/1 M KCl reference electrode
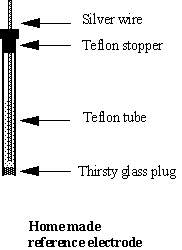
The reference electrode consists of a silver wire (F ~ 1 mm, length ~ 10 cm) coated with AgCl and dipped into a 1 M KCl solution. The compartment is a Teflon tube (ID = 2.5 mm, OD = 4 mm) with a “thirsty glass” plug (length ~ 3 mm) and a tight Teflon stopper on the top.
Procedure for coating the silver wire with AgCl:
Clean the silver wire with a fine grade emery paper and rinse it with distilled water. Insert the part to be coated into 0.1 M HCl and pass 10 mA/cm2 for about 1 min, using a platinum wire as a cathode. During the electrolysis a black deposit is formed on the silver. Rinse the electrode with pure water and insert it into the Teflon tube compartment filled with 1 M KCl. Before use place the reference electrode in a beaker with 1 M KCl for a few hours to reach a stable potential.
When not in use store the electrode in 1 M KCl.
The potential of an electrode prepared as above is within 1 mV of the expected value.